If you’ve ever come across the cryptic expression “hcooch ch2 h2o” in chemistry-oriented blogs or forums, it likely refers to the hydrolysis of methyl formate (HCOOCH₃) by water. This deceptively simple reaction underlies key principles of organic chemistry and also holds industrial importance.
In this detailed article, we’ll explore:
- What “hcooch ch2 h2o” really means
- The chemical structure and properties of methyl formate
- The mechanism and kinetics of its hydrolysis
- Catalysts, conditions, and equilibrium challenges
- Industrial applications and process design
- Safety, environmental aspects, and future directions
By the end, you’ll understand why “hcooch ch2 h2o” is more than a random chemical string — it represents a reaction that bridges textbook chemistry and real-world production.
What Does “hcooch ch2 h2o” Represent?
The phrase hcooch ch2 h2o is not a standard chemical notation, but many blogs use it as a casual rendering of:
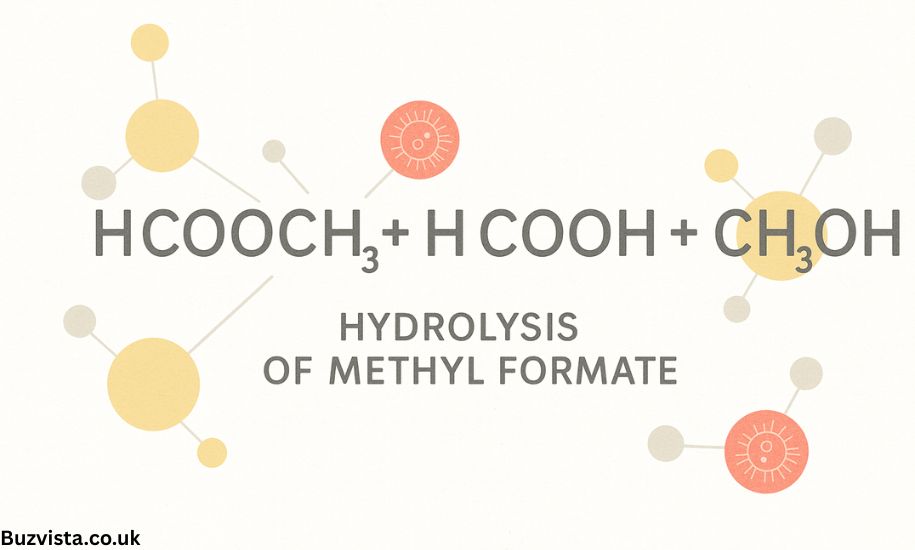
HCOOCH₃ + H₂O → HCOOH + CH₃OH
In other words:
- HCOOCH₃ = methyl formate
- H₂O = water
- HCOOH = formic acid
- CH₃OH = methanol
So, “hcooch ch2 h2o” represents the hydrolysis of methyl formate, an ester that reacts with water to produce formic acid and methanol. The process is both reversible and catalyzed by acid or base.
Structure, Properties & Reactivity of Methyl Formate
Molecular Structure
Methyl formate is the methyl ester of formic acid (HCOOH). Structurally, a formyl group (H–C=O) is connected via an oxygen atom to a methyl group (–OCH₃). This makes the molecule polar, reactive at the carbonyl carbon, and prone to nucleophilic attack.
Physical and Chemical Properties
- Appearance: Colorless, highly volatile liquid
- Boiling point: Around 31–32 °C
- Odor: Fruity, characteristic ester smell
- Density: About 0.97 g/cm³ at 20 °C
- Solubility: Miscible with most organic solvents and moderately soluble in water
Because of its low boiling point and ester bond, methyl formate reacts easily with nucleophiles such as water, alcohols, and amines.
Reactivity Overview
The carbonyl carbon in the ester linkage is electrophilic, meaning it readily attracts electron-rich species like water. Under the right conditions, water attacks this carbon, breaking the ester bond and forming an alcohol (methanol) and a carboxylic acid (formic acid).
The HydrThe Hydrolysis Reaction: Mechanism and Kinetics
Basic Reaction Equation
The central reaction is:
HCOOCH₃ + H₂O → HCOOH + CH₃OH
This equation encapsulates the entire “hcooch ch2 h2o” topic — a classic example of ester hydrolysis.
Mechanism — Acid-Catalyzed Pathway
Under acidic conditions, the hydrolysis of methyl formate proceeds through a well-understood sequence:
- Protonation of the carbonyl oxygen:
The carbonyl oxygen is protonated by an acid catalyst, increasing the electrophilicity of the carbonyl carbon. - Nucleophilic attack by water:
Water molecules attack the carbonyl carbon, forming a tetrahedral intermediate. - Proton transfers within the intermediate:
The intermediate undergoes internal proton shifts to stabilize the leaving group. - Cleavage of the C–O bond:
The methoxy group (–OCH₃) departs, forming protonated methanol. - Deprotonation of the product:
Deprotonation yields formic acid (HCOOH) and regenerates the acid catalyst.
This pathway is reversible, and the equilibrium can favor reactants or products depending on conditions such as water concentration and temperature.
Base-Catalyzed Pathway
Under basic conditions (saponification-type reaction):
- A hydroxide ion (OH⁻) attacks the carbonyl carbon directly, forming a tetrahedral intermediate.
- The intermediate collapses, expelling the methoxide ion (CH₃O⁻).
- Proton transfer from water converts CH₃O⁻ into methanol and leaves behind the formate ion (HCOO⁻).
- After acidification, the final product is formic acid.
This route is typically irreversible, because the formate ion is stabilized by resonance and does not easily revert to the ester.
Reaction Kinetics and Equilibrium
- Equilibrium Constant (K): The reaction is not highly product-favored (typical K values range around 0.2 under standard conditions).
- Le Chatelier’s Principle: To maximize yield, excess water or removal of methanol can shift the equilibrium toward formic acid formation.
- Temperature: Moderate heating (around 90–140 °C) accelerates the reaction rate.
- Catalyst Concentration: Higher acid concentration increases the rate but may cause side reactions if too strong.
Because the reaction is equilibrium-limited, continuous removal of methanol or using a large excess of water is often necessary in industrial settings to drive it forward.
Catalysts, Conditions, and Process Design
Catalysts
- Acid Catalysts
- Commonly used acids: sulfuric acid, hydrochloric acid, p-toluenesulfonic acid.
- Their role: protonate the carbonyl oxygen to activate the ester bond for nucleophilic attack.
- Base Catalysts
- Sodium hydroxide or potassium hydroxide can be used in saponification processes.
- They offer faster reaction rates but produce formate salts, requiring post-neutralization.
- Solid or Heterogeneous Catalysts
- Zeolites, ion-exchange resins, and supported acids are explored to simplify product separation and reuse catalysts.
Operating Conditions
- Temperature: 90–140 °C typical in continuous systems.
- Pressure: Slightly elevated to keep water and methanol in liquid phase.
- Water-to-ester ratio: Large excess of water ensures complete conversion.
- Residence time: Adjusted to balance yield and throughput.
- Continuous operation: Multi-stage or reactive distillation setups are favored for efficient equilibrium management.
Industrial Process Design
In commercial production, methyl formate hydrolysis is often integrated with formic acid synthesis:
- Methanol and carbon monoxide react to form methyl formate (carbonylation step).
- Methyl formate is hydrolyzed to yield formic acid + methanol.
- Methanol is recovered and recycled to the carbonylation stage.
This cyclic process minimizes waste and reagent consumption, improving both economics and sustainability.
Challenges include:
- Separation of methanol, water, and formic acid due to azeotropes.
- Controlling reversibility to prevent re-esterification.
- Managing energy costs in distillation and recycling steps.
Applications and Importance of the Reaction
1. Production of Formic Acid
Formic acid is a versatile industrial chemical used in:
- Textile dyeing and finishing
- Leather tanning
- Agriculture (as a silage preservative and antibacterial agent)
- Cleaning agents and de-scaling solutions
- Chemical synthesis (reducing agent, hydrogen source)
Because of its growing demand, efficient hydrolysis of methyl formate remains an important route to sustainable formic acid production.
2. Methanol Recovery and Utilization
Methanol, the byproduct of hydrolysis, is:
- A widely used solvent
- A raw material for formaldehyde, acetic acid, and methyl esters
- An alternative energy carrier in methanol-based fuel technologies
Since methanol is both the reactant (in methyl formate synthesis) and the product (after hydrolysis), it can be recycled, reducing waste and improving cost efficiency.
3. Educational Significance
The reaction HCOOCH₃ + H₂O → HCOOH + CH₃OH is one of the simplest yet most illustrative examples in organic chemistry classes. It demonstrates:
- Ester hydrolysis principles
- Acid/base catalysis mechanisms
- Equilibrium and kinetic behavior
- Industrial process translation from lab to plant scale
Because it is clean, well-understood, and representative of broader ester chemistry, it’s frequently used in teaching and demonstrations.
4. Environmental and Green Chemistry Perspective
Methyl formate hydrolysis exemplifies green chemistry concepts:
- Water as a benign reagent (no toxic byproducts)
- Minimal waste generation
- Potential for closed-loop operation
- Use of recyclable methanol
- Lower toxicity compared to other formic acid production routes
Modern research focuses on improving energy efficiency and developing solid catalysts to avoid corrosive acids or bases, aligning with sustainability goals.
Safety and Handling Considerations
While this reaction involves relatively simple chemicals, each component demands careful handling.
Methyl Formate
- Flammable and volatile.
- Vapors can cause dizziness or irritation.
- Handle in well-ventilated areas away from ignition sources.
Formic Acid
- Corrosive and can cause burns on contact.
- Inhalation of vapors may irritate respiratory passages.
- Requires gloves, goggles, and protective clothing.
Methanol
- Highly flammable and toxic if ingested or absorbed through the skin.
- Exposure can cause headaches, nausea, or even blindness at high doses.
- Store in tightly closed containers away from heat.
General Precautions
- Use fume hoods or ventilated areas.
- Wear personal protective equipment.
- Ensure emergency showers and eyewash stations are accessible.
- Dispose of waste according to chemical safety guidelines.
Environmental and Industrial Challenges
- Energy Consumption:
Distillation and product purification consume significant energy. Optimizing heat integration and recycling can mitigate this. - Azeotropic Separation:
Formic acid–water and methanol–water mixtures can form azeotropes, complicating purification. Specialized distillation or extraction is required. - Corrosion Issues:
Acidic conditions can corrode metallic equipment. Selecting proper materials (e.g., stainless steel, glass-lined reactors) is vital. - Emission Control:
Volatile organic compounds (VOCs) like methyl formate and methanol must be contained to minimize environmental release. - Wastewater Management:
Treatment of process water is essential to comply with environmental regulations.
Future Prospects and Research Directions
The “hcooch ch2 h2o” reaction continues to attract research attention for both academic and industrial reasons. Key focus areas include:
- Advanced Catalysis:
Development of solid acid catalysts, ionic liquids, and nano-catalysts to replace traditional liquid acids. - Process Intensification:
Using reactive distillation, membrane reactors, or microreactor technology to enhance conversion and energy efficiency. - CO₂ Utilization Link:
Integrating methyl formate hydrolysis with carbon dioxide–to–formate or methanol cycles, promoting carbon capture and reuse. - Renewable Feedstocks:
Employing bio-methanol or renewable CO₂ sources to make the entire process sustainable. - Computational Modelling:
Using kinetics simulations and process modeling to optimize conditions and reactor designs.
The intersection of chemistry, engineering, and environmental science makes this a continually evolving area of innovation.
Summary and Final Thoughts
In essence, “hcooch ch2 h2o” stands as shorthand for the hydrolysis of methyl formate, a reaction that beautifully merges theory and practice. It demonstrates:
- The interplay of mechanism and catalysis
- The reversibility and control of equilibrium reactions
- The industrial relevance of fundamental organic chemistry
Beyond its classroom significance, this reaction underpins a major industrial route to formic acid, one of the simplest yet most useful organic acids. As industries shift toward greener, circular processes, improving the hydrolysis of methyl formate remains an important target for sustainable chemistry.
Thank you for reading this comprehensive guide on hcooch ch2 h2o. For more insightful chemistry and science content, visit Buz Vista — your window into the world of chemistry and innovation.